Commodity Production Costs Report
Solid NaCN Production from Caustic Soda, Ammonia and Natural Gas
Sodium Cyanide Plant Capital & Operating Cost Analysis | United States | Q4 2025
This report presents the economics of Sodium Cyanide production from ammonia, natural gas, and caustic soda. The process examined is a typical Andrussow process. In this process, high purity hydrogen cyanide is initially produced via a direct synthesis method involving the reaction of ammonia, methane (natural gas), and air. Subsequently, the hydrogen cyanide and sodium hydroxide solution (50 wt%) react and water is evaporated. Solid Sodium Cyanide is obtained as the final product.
The report provides a comprehensive study of Sodium Cyanide production and related Sodium Cyanide production cost, covering three key aspects: a complete description of the Sodium Cyanide production process examined; an in-depth analysis of the related Sodium Cyanide plant capital cost (Capex); and an evaluation of the respective Sodium Cyanide plant operating costs (Opex).
The Sodium Cyanide production process description includes a block flow diagram (BFD), an overview of the industrial site installations, detailing both the process unit and the necessary infrastructure, process consumption figures and comprehensive process flow diagrams (PFD). The Sodium Cyanide plant capital cost analysis breaks down the Capex by plant cost (i.e., ISBL, OSBL and Contingency); owner's cost; working capital; and costs incurred during industrial plant commissioning and start-up. The Sodium Cyanide plant operating costs analysis covers operating expenses, including variable costs like raw materials and utilities, and fixed costs such as maintenance, labor, and depreciation.
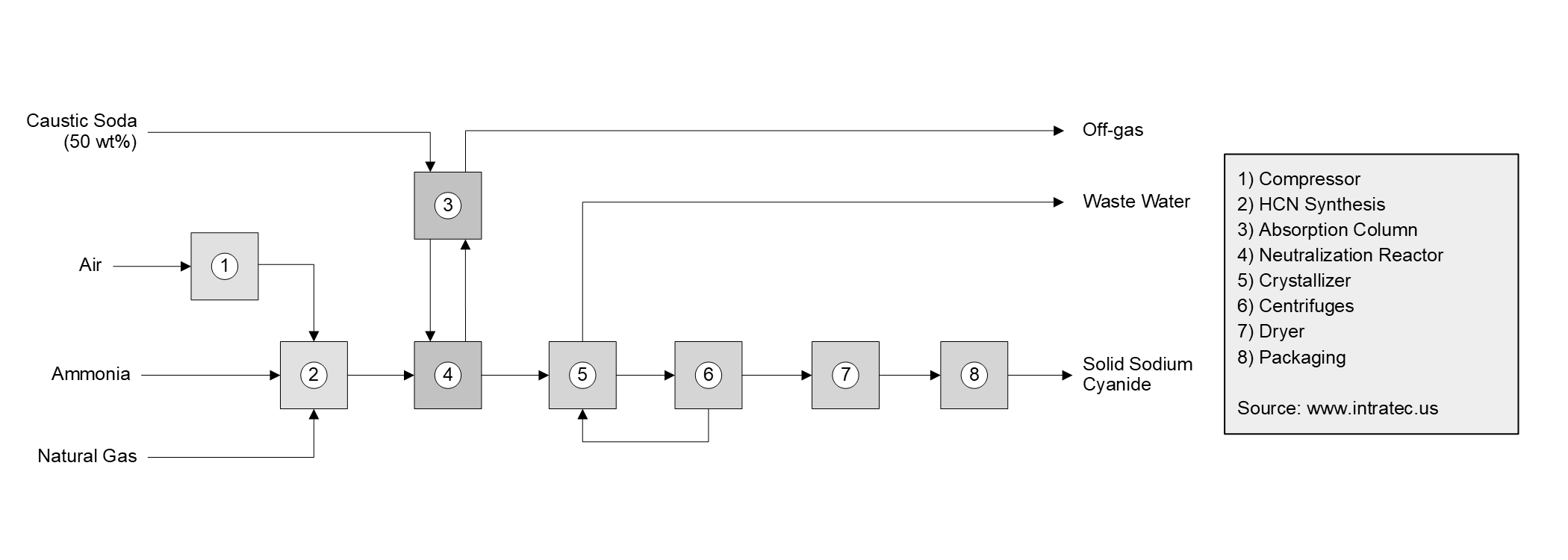
The process under analysis comprises three major sections: (1) HCN Production; (2) Neutralization; and (3) Purification.
HCN Production. Hydrogen cyanide is produced through a direct synthesis method similar to the Andrussow process. Natural gas, ammonia, and air are fed to a reactor in the presence of a platinum-rhodium catalyst, where HCN is formed through a catalytic reaction. The product stream is subjected to subsequent purification steps, to produce a 99.5% HCN stream.
Neutralization. First, caustic soda is fed to an absorption column to absorb hydrogen cyanide from the vapor stream that exits the neutralization reactor. The sodium hydroxide solution is then fed to the neutralization reactor alongside hydrogen cyanide from the previous section. While the reaction is exothermic, the reaction heat is removed by means of circulation through an external heat-exchanger, in such a way that the solution temperature is kept below 50 °C. Yields greater than 98% are achieved based on HCN. At the end of this step, a salt solution of NaCN (around 45 wt%) is obtained.
Purification. The salt solution is fed to evaporative crystallizers, in which water is removed to reach the saturation concentration and begin the crystallization. The sludge from the crystallizer is then fed to a set of centrifuges to separate the Sodium Cyanide crystals. The mother liquor recovered from the centrifuges still contains about 40 wt% in Sodium Cyanide, so it is recycled to the crystallizers. Finally, residual moisture in the crystals is removed in a spin flash dryer using hot air. The dried solid powder is then compacted in hydraulic roll presses into briquettes for safer handling, which are then packaged into storage bins and directed to storage.
Report in PDF Format
Download & Explore Anytime
Access in Various Devices
Print & Read Comfortably
Share With Co-workers
Up-to-date Report
Professional report based on Q4 2025 economic data, ensuring timely evaluations.
Multiple Use Cases
Ideal for investment screening, feasibility studies, cost estimates, and research planning.
Proven Methodology
Developed using a consistent methodology honed over a decade, ensuring reliable cost analyses.
Report Editions
Content Highlights
Plant Capital Cost Summary
Summary outlining the capital cost required for building the Sodium Cyanide production plant examined.
Plant Capital Cost Details
Detailing of fixed capital (ISBL, OSBL & Owner’s Cost), working capital and additional capital requirements.
Plant Cost Breakdowns
Breakdown of Sodium Cyanide process unit (ISBL) costs and infrastructure (OSBL) costs; plant cost breakdown per discipline.
Operating Costs Summary
Summary presenting the operating variable costs and the total operating cost of the Sodium Cyanide production plant studied.
Operating Cost Details
Detailing of utilities costs, operating fixed costs and depreciation.
Plant Capacity Assessment
Comparative analysis of capital investment and operating costs for different Sodium Cyanide plant capacities.
Production Process Information
Block Flow Diagram, descriptions of process unit (ISBL) and site infrastructure (OSBL).
Process Consumptions
Raw materials and utilities consumption figures, by-products credits, labor requirements
Process Diagrams
Process flow diagrams (PFD), equipment list and industrial site configuration
Other Sodium Cyanide Production Cost Reports

Solid NaCN Production from Sodium, Charcoal and Ammonia
This report examines the costs related to Sodium Cyanide production from metallic sodium, charcoal and ammonia. In this process, molten sodium, ammonia, and charcoal react to form Sodium Cyanide. Solid Sodium Cyanide is obtained as the final product. The economic analysis provided assumes a plant located in the United States.
Details: 0.8 kta United States-based plant | Q4 2025 | 107 pages | Issue A | From $999 USD

Solid NaCN Production from Soda Ash, Charcoal and Ammonia
This report presents the economics of Sodium Cyanide production from sodium carbonate, charcoal and ammonia in the United States. In this process, soda ash, ammonia, and charcoal react to form Sodium Cyanide. Solid Sodium Cyanide is obtained as the final product.
Details: 0.8 kta United States-based plant | Q4 2025 | 107 pages | Issue B | From $999 USD

Solid NaCN Production from Caustic Soda and HCN
This report provides a techno-economic analysis of Sodium Cyanide production from hydrogen cyanide and caustic soda. In this process, hydrogen cyanide and sodium hydroxide solution react and water is evaporated. Solid Sodium Cyanide is obtained as the final product. The economic analysis provided assumes a plant located in the United States.
Details: 45 kta United States-based plant | Q4 2025 | 107 pages | Issue D | From $1,199 USD
Bundle & Save
Purchase multiple Sodium Cyanide Production Cost reports and enjoy tiered discounts
up to 30% off!
Could Not Find the Report You Need?
Obtain a Bespoke Report
Get a report targeting the process in which you are interested
See Offer Details
Understand Bespoke Reports and how you can easily order them
Check Editions & Pricing
Complete a brief form and see a quotation for your Bespoke Report
Other Related Production Cost Reports

Ammonia Production from Natural Gas (Combined Reforming)
This report presents the economics of Ammonia production from natural gas in the United States using a process similar to KBR Purifier process. In the process examined, syngas is initially produced from natural gas via a combined reforming. The syngas is purified through several steps into nitrogen and hydrogen which are synthesized to ammonia.
Details: 800 kta United States-based plant | Q4 2025 | 107 pages | Issue B | From $1,199 USD

Sodium Hydroxide Production from Sodium Chloride (Diaphragm Process)
It presents the economics of Sodium Hydroxide production from brine in the United States. The process examined in this report is a typical diaphragm process. Chlorine and hydrogen are also generated as by-products in the process.
Details: 600 kta United States-based plant | Q4 2025 | 107 pages | Issue B | From $799 USD

Ammonia Production from Syngas
This report presents the economics of Ammonia production from synthesis gas (syngas). In this process, hydrogen, recovered from syngas feedstock, and nitrogen, recovered from air, react producing Ammonia. The economic analysis performed assumes a plant located in the United States.
Details: 800 kta United States-based plant | Q4 2025 | 107 pages | Issue A | From $799 USD

Sodium Hydroxide Production from Sodium Chloride (Membrane Process)
This report presents the economics of Sodium Hydroxide production from sodium chloride in the United States, via a typical membrane process. In this process, sodium chloride is decomposed electrolytically, producing sodium hydroxide. Chlorine and hydrogen are generated as by-products in the process.
Details: 550 kta United States-based plant | Q4 2025 | 107 pages | Issue A | From $799 USD
+800 Reports Developed, Targeting +250 Commodities
Vast Report Library
858 independent and up-to-date reports examining embryonic and established production processes.
Free Sample Reports
Quickly understand the structure and depth of content of our professional reports.

